This is a great sample for contrast in mechanical measurement techniques like PeakForce QNM, force volume, and so on. And it is a good representative of a soft sample that is well suited to PeakForce imaging where the peak normal force is known and controlled with minimal shear force. This allows for imaging under different peak normal forces, which can lead to different surface structures just due to deformation of the surface and interaction with subsurface structures.
The top image is a height image taken with a peak normal force of 5 nN using a fairly stiff probe, a silicon SCM-PIT which has a spring constant of around 3 nN/nm. Deflection sensitivity was calibrated on sapphire, and the spring constant was estimated through thermal tuning to be around 2.1 nN/nm. This is stiff enough of a probe that the Sader method is more appropriate.
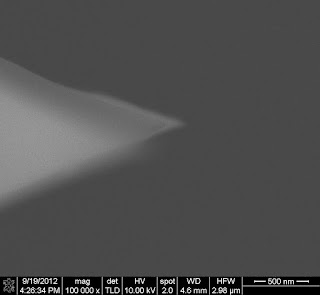
Since the SCM-PIT is coated with PtIr for EFM and KPFM, it was possible to do KPFM to image the surface potential. Initially this sample was chosen to calibrate the probe radius to simultaneously do QNM and KPFM, to correlate both nanomechanical properties and surface potential properties of a different sample.
But-- why not KPFM on a soft biphasic polymer?
The bottom image is the raw PeakForce KPFM channel. The lift was 50 nm with a scan-rate of 0.1 Hz. A three hour image! What is immediately obvious is that the PDMS domains have a surface potential some 50-70 mV higher than the PS. There is also a potential "plateau" across the top left of the image, and athese little specs on some of the PS inclusions are clearly hitchhikers, contaminants, and they have a surface potential of 200-400 mV above the neighboring PS background.
But-- why not KPFM on a soft biphasic polymer?
The bottom image is the raw PeakForce KPFM channel. The lift was 50 nm with a scan-rate of 0.1 Hz. A three hour image! What is immediately obvious is that the PDMS domains have a surface potential some 50-70 mV higher than the PS. There is also a potential "plateau" across the top left of the image, and athese little specs on some of the PS inclusions are clearly hitchhikers, contaminants, and they have a surface potential of 200-400 mV above the neighboring PS background.